Hidden Pitfalls in the Risk Management Strategy
In our fast-paced world, the risks we have to take and manage, in order to continue to grow and to develop, evolve quickly. Effectively managed risks help companies achieve their goals.
Products that are developed following quality standards, have improved the quality of life for thousands of people. The idea of improving the quality of life is the very premise of product risk management.
Risk is About Context
Risk management is one of the key factors in quality management. Various standards include Risk Management procedures, but they leave enough space to be creative and adaptive: there is no step-by-step guide as to how to prepare the risk management plan, nor an exact description on how to implement or document risk management activities, although ISO14971 does provide guidance.
The main reason for such a wide range of flexibility is the diversity of needs and different cases to meet the individual needs of each company. This is why it’s easy to find so many different risk management approaches, templates, guides, advice, etc. on the internet. It also explains why information in different blogs might be contradictory, but almost any risk management strategy might work as long as it’s appropriate for the company’s specific needs.
Hidden Pitfalls in the Risk Management Strategy
If the risk management strategy is not properly prepared, or if it’s based on inaccurate directives, or if the lack of experience distorts the planning of hazard evaluation, etc., these could inevitably lead to undesired results in managing risks. Ironically, the risk is that these will complicate the process of identifying real hazards, therefore some of the most relevant risks could stay undefined and ignored, and precious company resources (attention, effort, money…) wasted on irrelevant hazards.
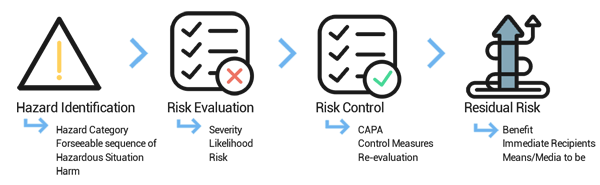
Proper Risk Management Plan
The Risk Manager needs the right experience in evaluating risks, or at least to have the right guidance on how to prepare the risk management plan, how to organize risk management training, how to measure if the team members of the management group are well prepared to efficiently identify real hazards in their department. Although it might seem like just simple common sense, it is very important to use a companywide, unified risk management strategy for evaluating and controlling risks. The risk management outputs have to be documented thoroughly and appropriately handled for further processing and implementing CAPA actions.
Effectively managed risks help companies achieve their goals. qmsWrapper Risk Management is a powerful, yet user-friendly tool, that makes risk & compliance management easy for everyone.
qmsWrapper makes possible for risk or quality managers even with little experience to appropriately perform their risk-related tasks.
qmsWrapper has an integrated approach to managing risk across an organization.
It’s flexible so it can be appropriate and proportionate to the complexity and type of
organization involved.

It can be integrated into the design process from the get-go, then revisited at every phase.
All the necessary information, like the hazardous situation at risk identification phase, the hazard categories are on a single interface, graphically mapped out for a simple overview.
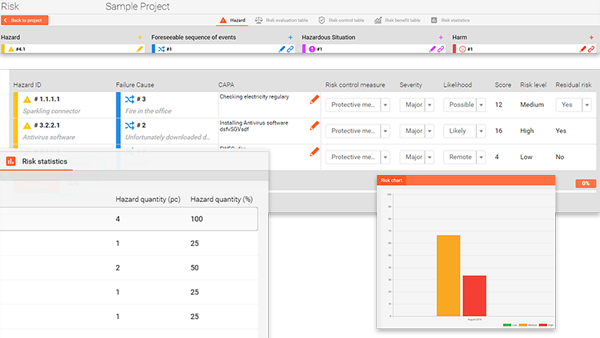
Some level of failure is inevitable, but the use of a smart risk management system can help to mitigate the frequency and severity of failures.
The software is based on ISO 14971 guidance and combined with a flexible system where you can create your own Risk processes, including approval workflows, and define your own risk varieties and action plans.
qmsWrapper Risk Management provides a step by step sequence of task with appropriate explanations and cautions that makes possible for risk or quality managers even with little experience to appropriately perform their risk-related tasks.
Your risk controls will have a direct impact on your design outputs, as well as your verification and/or validation activities. An inappropriate risk management strategy will lead to incorrect hazard evaluation that will end in wasted resources.
If you’re ready to get serious about your risk management and product development efforts, qmsWrapper is your solution.
Cup of Joe #47 - 2 Risk Modules
The New Risk Module supports risk-based design
Wrap Jira in QMS