Create
any project
successfully!

Control your
submission
documentation.

Ensure
regulatory
compliance.

ISO 13485, CE Mark
21 CFR Pt.11, 21 CFR 820,
FDA 510k.
More than +500 companies trusted
qmsWrapper with their QMS.
qmsWrapper with their QMS.

Elevate Your Quality Management
with qmsWrapper
with qmsWrapper
qmsWrapper provides powerful tools to manage critical quality processes, including CAPA, Nonconformity, Training, Change Management, Supplier Management, and Feedback. Our robust modules—Project Management, Quality Management, Document Management, Team Communication, Traceability Matrix, and Risk Management—are designed to enhance compliance and operational efficiency.
qmsWrapper integrates seamlessly into your workflow, allowing for consistent oversight and control across all quality processes. Whether you're handling routine tasks or addressing complex challenges, our platform supports your team every step of the way, ensuring nothing falls through the cracks.

Reliable
Trustworthy software to use - validated
according to ISO/TR 80002-2
according to ISO/TR 80002-2

Easy to implement and use
Out-of-the-box ready for use with
included default SOP templates and
workflow processes.
included default SOP templates and
workflow processes.

Fully Scalable
Implement the modules at your own
place, so your quality system can grow
with your team.
place, so your quality system can grow
with your team.
Choose your eQMS
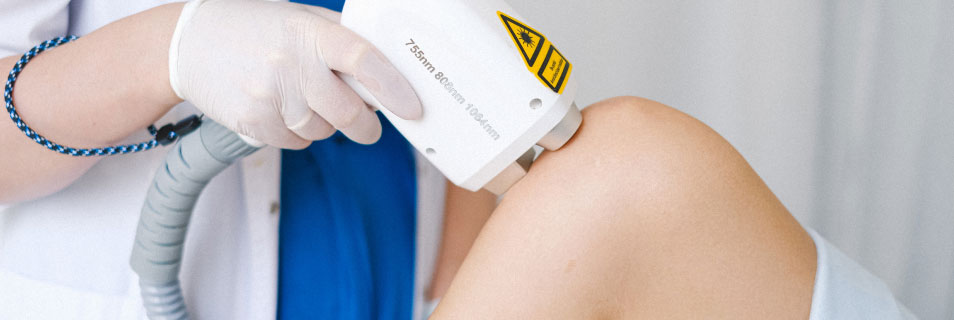
eQMS for MedDev ISO 13485
qmsWrapper is an affordable and unique solution for start-up and small-sized companies involved in the medical device industry. qmsWrapper is envisioned as a tool to facilitate the implementation of a quality management system by establishing document control management, design controls, risk management, and other activities related to quality.

eQMS ISO 9001
qmsWrapper is envisioned as a tool to facilitate the implementation of quality management systems by offering different features, such as document control management, electronic signature, design controls, risk management module, and many more. We believe in MTQ (Management through quality) where anyone can tailor each project to fit each individual business. qmsWrapper, as best-in-class, can provide result-orientated collaborative QMS compliance since it designed to help electronically implement and maintain ISO 9001 compliance activities.
Tailored Features
for Your Industry Needs
for Your Industry Needs
At qmsWrapper, we understand that each industry has its unique quality management requirements. That's why our software offers a range of features tailored to meet the needs of businesses operating in different sectors:
- Compliance Made Easy: Ensure compliance with industry standards such as ISO 13485 for medical devices and ISO 9001 for general quality management. qmsWrapper helps you establish and maintain a robust quality management system that meets regulatory requirements effortlessly.
- Risk Management Simplified: Simplify risk assessment and management processes with our integrated tools, ensuring compliance with standards like ISO 14971 for medical devices. Identify, assess, and mitigate risks effectively to protect your products and reputation.
- Document Control: Maintain complete control over your documents with our intuitive document management system. Organize, review, and approve documents with ease, enhancing document traceability and version control.
- Cloud-Based Accessibility: Access your quality management system anytime, anywhere with our cloud-based platform.
- Electronic QMS: Say goodbye to paper-based processes and embrace the efficiency of electronic quality management. qmsWrapper digitizes your QMS, allowing for faster approvals, real-time updates, and much more.
- Audit Management: Prepare for audits with confidence using qmsWrapper's audit management tools. Schedule audits, track findings, and generate comprehensive reports to demonstrate compliance.

Let’s Get The Conversation Started
Contact us today to arrange a free consultation
with one of our qmsWrapper experts.
with one of our qmsWrapper experts.

Software
presentation
presentation
12 integrated
features
features

What do our clients
say about us?
say about us?

Partner with
qmsWrapper
qmsWrapper