Approval Workflows in QMS
Issues in approval workflows
Achieving and maintaining compliance is not an easy job as both ISO and FDA regulations require that documents be approved before they are officially distributed or used either inside or outside the company. Obtaining approval from a group of people for a project plan, a proposal, or any other required document can be a frustrating experience:
First, there is the issue of formally recording and keeping track of which people approved which document. Then there is the issue of managing the logistics of getting the document to the right people without getting tongue-tied in excessive email threads to share your collected data or even scheduling meetings and trying to save the meeting notes. Chasing your coworkers to sign off on a project or repeatedly emailing and asking them to review that data you sent a week ago, is exhausting and annoying work.
To routinely manage the approval or review for important compliance documentation is not something that should be done on an ad-hoc basis – that just won’t do the job...
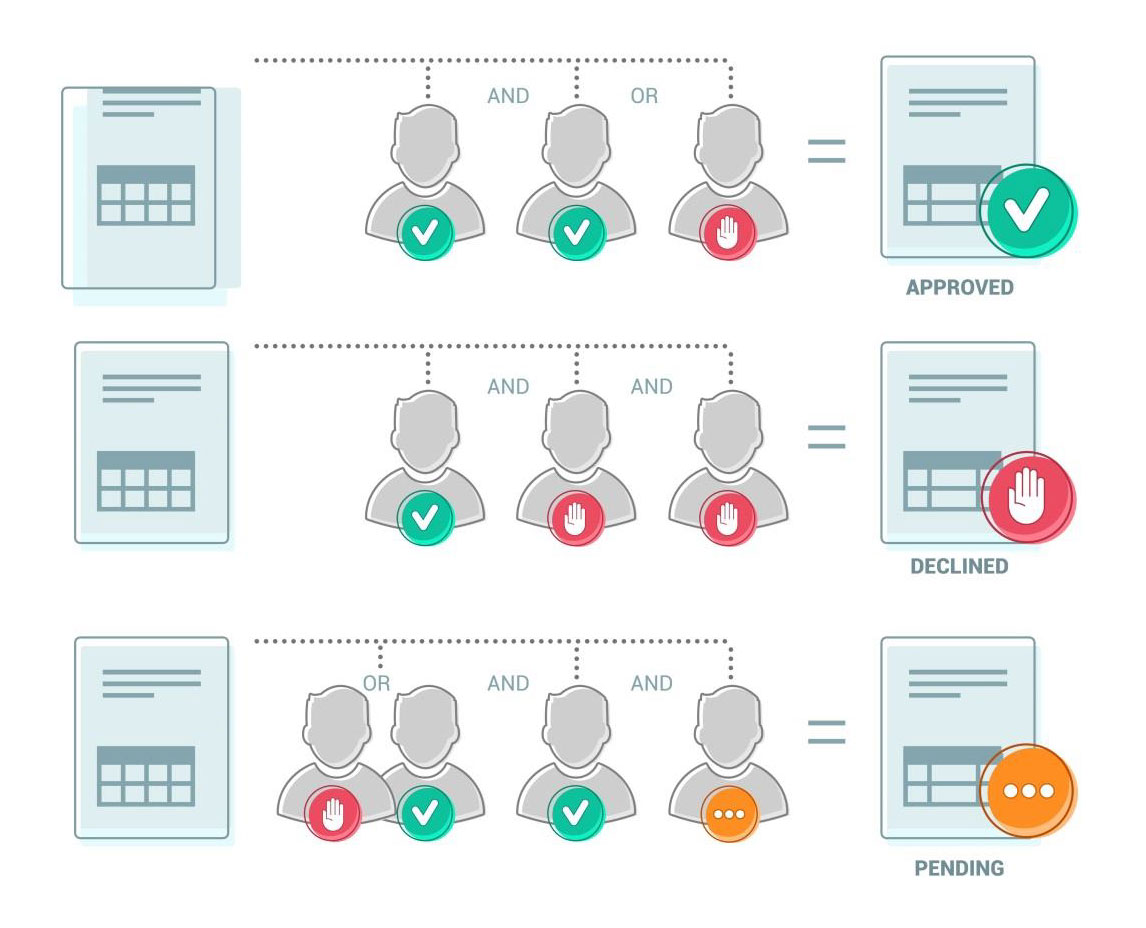
Why do we need QMS Approval Processes?
Approval processes are supporting the accurate recording of acceptance or agreement in business documents, policies, work instructions, handbooks, and more. They can add value to an existing creative or business workflows with creating consistency by always requiring approval when certain conditions are met, instead of relying on human memory to remember when and from whom to seek approval.
It is a fact that paper-based QMS systems are not effective, so, today many companies use business workflow software to automate approval workflows. But electronic-based systems are not necessarily a guarantee for easing the managers' work since managers are obliged to find, download, save, open, read, then print and sign, scan, upload, etc. each document. In the end, if there are too many documents to approve within a short time frame, the managers tend to be inclined to overview documents only superficially and approve them anyway, although they are not in the best shape, or worst, not even close to what was required.
Consider all the problems you could face weeks or months from now, from all these improper but approved documents, or misplaced files, emails lost or stored in wrong folders, or if you're unable to prove that you obtained approval from the right people.
However, there's a better way!
Improve Your Approval Process
The best practice shows that medical device companies are benefiting the most out of the software, where QMS is sewn into the fabric of workflows. These electronic tools are satisfying not just the company policies but also the various Standards requirements. Using the right software can streamline the cost and time required to coordinate common business processes. Using simplified workflow forms even the new, inexperienced employees can avoid making certain costly mistakes, the software can help ensure that employees cannot bypass established procedures.
qmsWrapper electronic Approval Workflow feature is designed to support business requirements for efficient reviews and help you automate your business processes, keeping compliance on track.
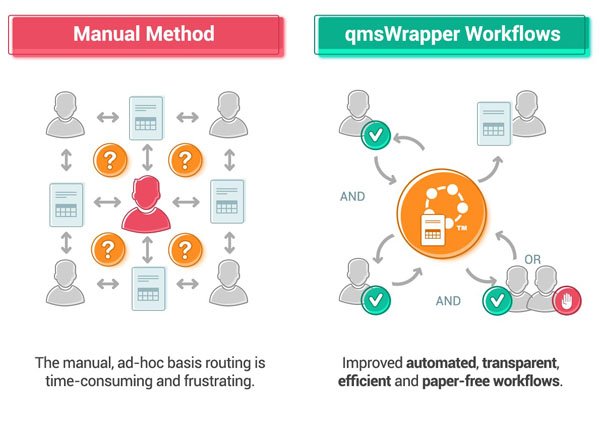
You can now concentrate on performing your work, qmsWrapper approval workflows assign tasks, send notifications, track participants, and creates a record of the entire process.
You can provide stakeholders with a link to the workflow history for the document, which shows who approved or rejected a document, or who failed to complete their workflow task.
“qmsWrapper makes the approval process more transparent, efficient and paper-free”
Document Approval Workflows is flexible and modifiable, you can choose to send the files for sequential or joined approvals:
- Sequential approvals are sending files to several users but with certain orders (after one user approves, the next will receive the document for approval). This method is useful for multilevel approvals in the organization structure. (For example: first, the unit manager approves ordering of something, then procurement approves it, and lastly, the CEO approves the document, all attached in one step).
- Joined approvals are sending documents for approval to a group of users (One document will be sent to several users at the same time, and it will be approved only when all of them approve it).
- Or both combined…
The workflow automates, streamlines, and standardizes the whole process:
- Predefined approval workflows – qmsWrapper includes a number of predefined workflows that address common business processes
- Editable workflows - Based on the settings that you specify when adding a workflow, you can either use the already existing ones or modify them or create unique versions, each with its own specialized way of working.
- Automatic notifications - Automatic reminders can speed up the approval processes by sending notifications to participants when needed.
- Data Safety - The task or document in need of approval is frozen during submission. Version history is always available, documents are being signed electronically with a user unique PIN code, just like your bank card. Review workflows are separate and more flexible than Approval Workflows.
- A Review workflow gives the option to send a file to various people and allows them to edit and modify any document. This allows for getting buy-in and peer review before a document is sent for Approval.
- Approval Workflow History Records - As files are electronically routed, approvals are recorded in the system. The history of submission events in a workflow run is never deleted from the database, meaning all data is protected for the future. Everything is recorded in an audit trail that can be reviewed and analyzed in reports.
- Transparency – in qmsWrapper every change made on a document/issue is saved. The document/issue details show which items are final and approved, who made the approvals, and the complete document approval history.
- Track Approvals - When you use a workflow to manage the document approval process, the server manages all of the tasks. Managers can track, monitor and adjust the activity steps in a workflow.
- Fast and Efficient- Replace ad-hoc and inconsistent approvals that rely on paper or email.
- Less work for you - An Approval workflow saves you and your colleagues both time and trouble, and at the same time streamlines and standardizes your approval process.
CHECK THIS USE CASE: "How to Establish Approval Workflows in qmsWrapper?"
Approvals are a core component of workflows and a cornerstone of effective process management. Combined with version control, a complete history of prior approved documents can be maintained, which supports retention regulations and limits the legal liability.
Capture document approvals electronically with qmsWrapper and build confidence and trust across the team.
QMS Tags
Upgraded Dashboard and QMS Control
Wrapper File App – advanced editing and control of the documents