qmsWrapper eQMS for Medical Devices
for ISO 13485 & FDA-ready Quality Management.
Organize projects, simplify document control, manage risks and foster
team collaboration with ease.
Strengthen your Design History File (DHF) and Technical File management
while staying audit-ready with Event & Hazard Logs and configurable workflows.
Project & Quality Management
Smarter projects, stronger compliance
With qmsWrapper, Project and Quality Management work as one. Tasks, approvals and QMS processes are woven together, so projects run efficiently without adding extra bureaucracy. This approach is called Managing Through Quality (MTQ).
Pre-defined and customisable workflows help teams align with ISO 13485 and FDA QSR requirements. Using the Workflow Process Editor, you can adapt ready-made processes or design your own—ensuring SOPs are implemented consistently and automatically.
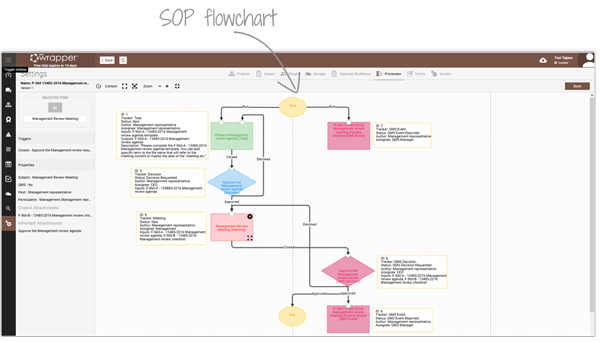
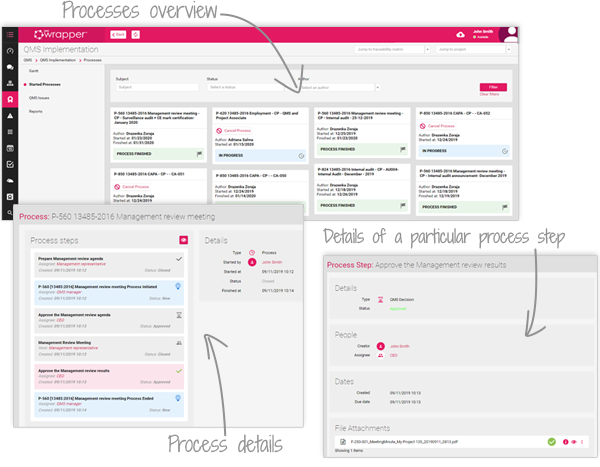
As tasks progress, the Event Log automatically records Changes, Deviations and Non-Conformities, giving managers real-time visibility across projects and QMS activities. The result: fewer errors, no missed reports, and complete audit-ready traceability.
Document Management
Compliance-ready control for every file
qmsWrapper’s Document Management System centralises all your files with version control, audit trail, tagging and secure Vault storage. Fast search and filters make it easy to find what you need and link documents directly to projects, risks or events.
Approval workflows and e-signatures (ERES) simplify reviews and keep full approval history, so audits move faster with no rework or missing records. Only authorised users can review and approve, ensuring compliance integrity.
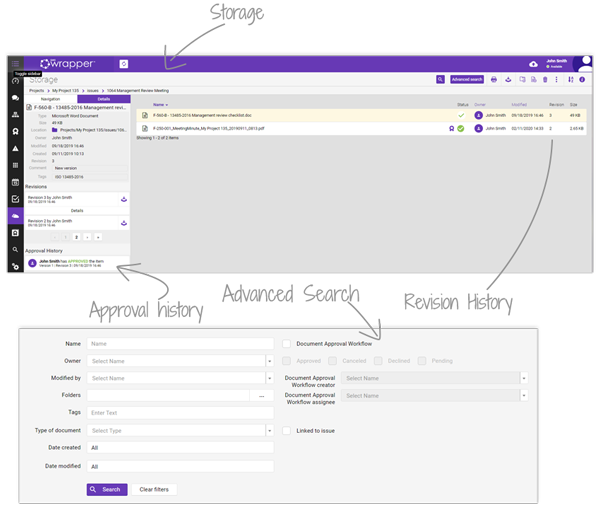

Access your documents anytime, from any device. Cloud-hosted storage keeps files secure yet always available—supporting FDA and ISO 13485 compliance while reducing the time teams spend chasing signatures or searching for the right version.
Risk Management
Control risks, strengthen compliance
qmsWrapper’s Risk Management module follows ISO 14971 guidance and gives teams a simple interface to identify, evaluate and control risks. By linking requirements, design inputs and verification activities, it ensures every decision is risk-aware and compliance-driven.
With the Hazard Log, all hazardous situations and categories are centralised, making risk assessments clear and traceable. Step-by-step workflows guide even less experienced managers through evaluations, reducing errors and missed actions.
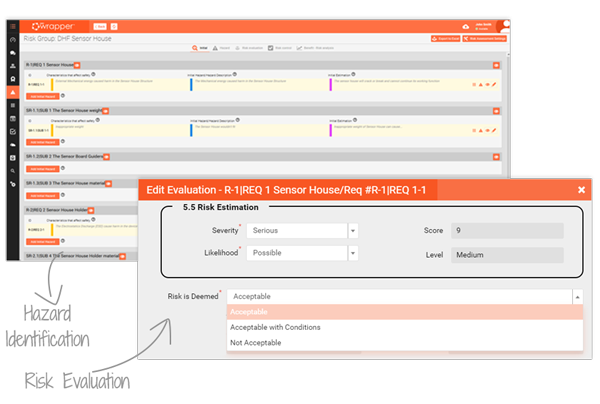
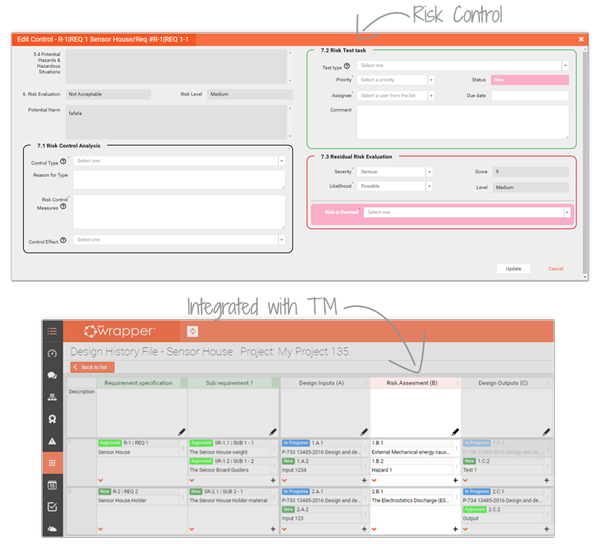
Integrated into the Traceability Matrix, risks are automatically connected to design controls, CAPA and validation tasks. The result: fewer failures, stronger audits, and a complete record of safety across your device lifecycle.
Form Editor
Custom forms that power your QMS
qmsWrapper’s Form Editor lets you design custom web forms to match your workflows or ISO 13485 requirements. From training records to CAPAs, every form can trigger actions, tasks or events, ensuring SOPs are implemented consistently.
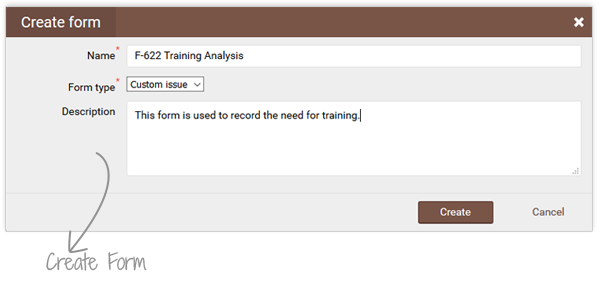
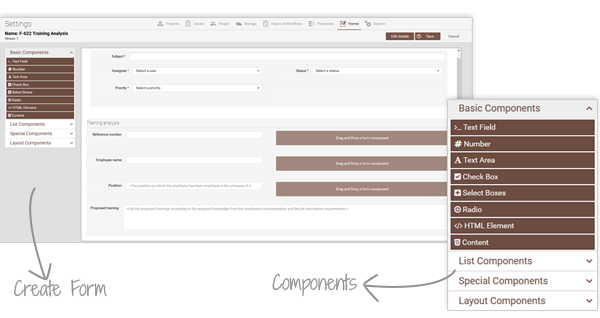
With drag-and-drop components, teams can create templates that adapt as regulations and business needs change. No more messy spreadsheets—just structured, automated forms that simplify compliance and save time.
Whether it’s documenting a deviation or recording training, forms become part of your QMS workflow, keeping data traceable, centralised and audit-ready.
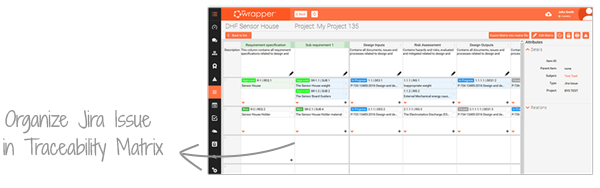
Traceability Matrix
End-to-end visibility for MedDev compliance
qmsWrapper’s Traceability Matrix gives you a clear view of your device lifecycle—from requirements and design inputs to verification, validation and risk analysis. Every change is linked and traceable, ensuring compliance with ISO 13485 and 21 CFR 820.
Built as a multi-user tool, the matrix lets your entire team collaborate in real time on the same file. Items such as forms, issues or processes can be added and traced through every stage, keeping projects aligned and transparent.
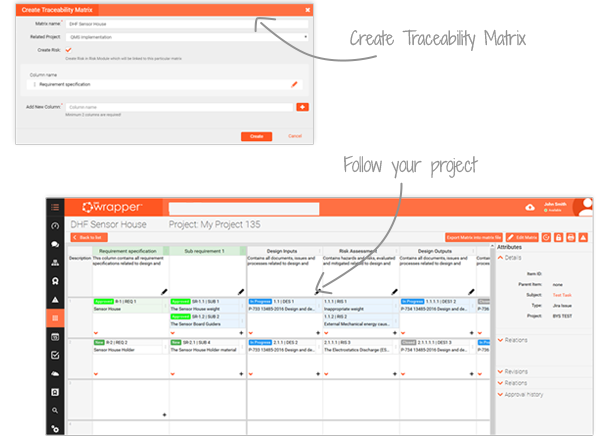
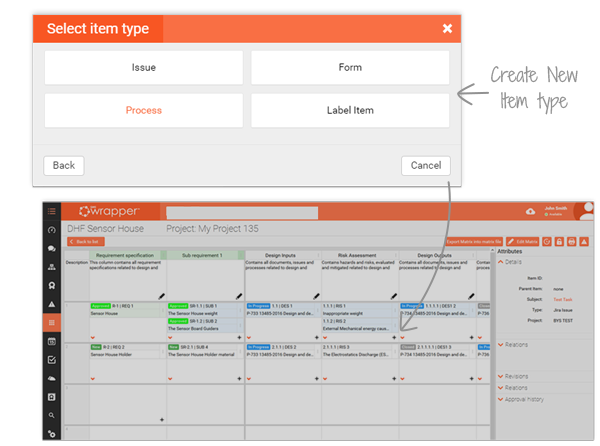
Seamlessly integrated with Jira, Document Management and Project Management, the Traceability Matrix becomes the backbone of your DHF and Technical File, supporting faster 510(k) and CE Mark submissions. Auditors can instantly see the full chain of evidence, saving your team days of preparation.
Integration with Jira
Better Control of Development and Compliance in One System
qmsWrapper integrates seamlessly with Jira, giving MedDev companies a unified environment to manage CE Technical Files and FDA Design History Files. Development tasks, design controls, and compliance records are connected in real time—helping teams stay agile and compliant without duplication of work.
With Jira inside qmsWrapper, you can track, assign, and review every task in one place. All Jira issues are visible within qmsWrapper’s Project Management and Traceability Matrix, ensuring end-to-end traceability and faster preparation for audits. Put simply, Jira is “wrapped with QMS”.
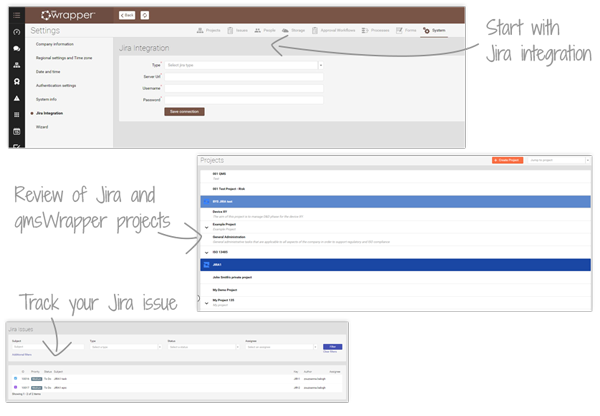
This integration reduces development risks, improves collaboration, and accelerates product delivery—while ensuring full alignment with ISO 13485 and FDA QSR requirements.
Team Messaging
Collaboration built for compliance
With qmsWrapper’s built-in chat, your team can have 1-on-1, group or topic-based conversations directly inside the QMS. No more switching between apps—communication, documentation and compliance stay in one place.
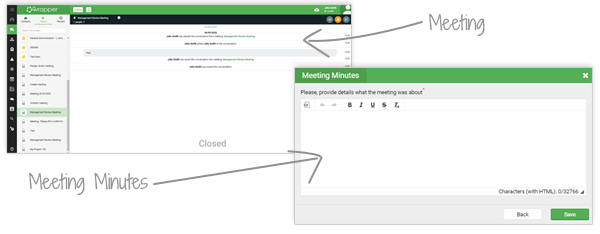
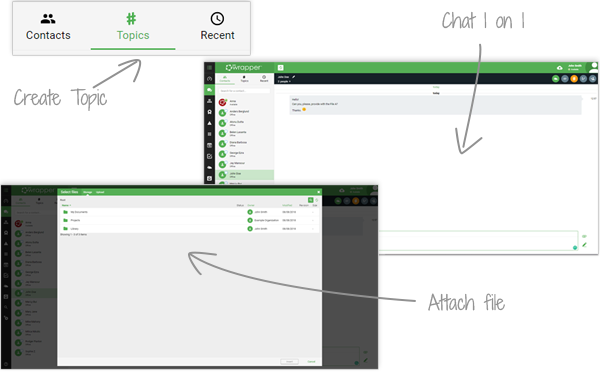
Chats can be saved as meetings, complete with agenda, minutes, scheduling and follow-up actions. Files shared in a chat are automatically stored in Document Management and linked to the right project or task.
By combining collaboration with compliance, qmsWrapper keeps teams productive and aligned with ISO and FDA requirements—whether they’re in the office or working remotely.
Contact us today to arrange a free consultation
with one of our qmsWrapper experts.