qmsWrapper Learning Hub
Master QMS compliance with practical resources. Explore expert-written whitepapers, demos, use cases, and step-by-step guides—kept current with ISO 9001, ISO 13485, and risk management best practices.

Download free, expert-written PDFs on remote audits, ISO 14971 risk, traceability matrix, eQMS validation, ISO 9001/13485 checklists, quality manuals, document control, and data security. Each paper includes practical checklists and examples—updated for Event & Hazard Logs where relevant.

Watch short demos and walkthroughs: Managing Through Quality, Document Management, Risk (ISO 14971 & ISO 9001), Traceability Matrix, plus use-case screencasts. Every video includes chapters and a transcript so you can jump to what matters.
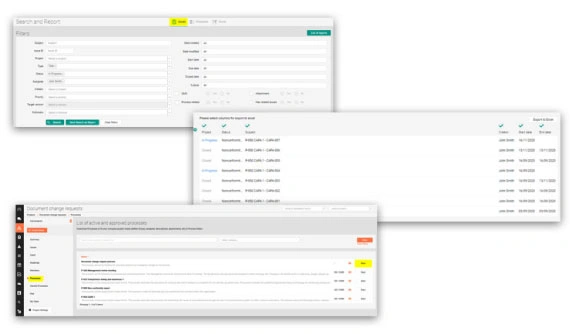
See real workflows in action—define roles, set approval workflows, record events, organize storage, and track revisions/approvals. Each use case shows the exact steps, expected outputs, and how to stay audit-ready.

15–30 minute guided paths that translate standards into daily practice. Start with Fundamentals, then dive into ISO 9001/13485, audits, event-driven QMS, traceability, and AI-ready workflows.

Step-by-step guidance for every feature with screenshots, tips, and linked resources. Use smart search to find answers fast and follow versioned, “last updated” pages for complete traceability.

News & Events
Stay current with release notes, webinars, and the “Cup of Joe” newsletter.
See what’s new in Event & Hazard Logs and get early looks at AI-ready resources.
See all news

A New Vision for Risk-Driven QMS with qmsWrapper

20 things you need to know about SOP for medical devices

