How are revision and approval history tracked in qmsWrapper?
Achieving and maintaining compliance is not an easy job as both ISO and FDA regulations require documents to be approved before they are officially distributed or used either inside or outside the company.
Document Management System is designed to support compliance, it includes version control, detailed file histories, file tracking, source tagging, comments, authority control. A document’s status is highlighted, so you can be confident that everyone is working on the most recent version.
Revisioning
Usually, a document or a record is not an outcome of one person, but several people in a team. Until its final approved version, it goes through several revisions which can be difficult to track. A good document management software (DMS) should be able to register and store all the revisions and versions that a user has worked on.
Here’s how revision control works using as an example SOP 004 CAPA Procedure:
The user that uploaded a file to the Storage becomes its owner. All members of a project or users that have permission to the file in a Library will be able to see it.
If a file should be changed, the user will download the file and start editing it. Once the file is completed, the user will save the changes and upload it back to the same location under the Storage by the same file name.
While uploading the new file revision, provide the comment about the updated file.
The new file revision will overwrite the previous one and the revision of the file is incremented to the next version, version 4.
Keep in mind the revisions are considered as drafts in qmsWrapper. Once the file gets approved, it will be considered as a version.
Approvals
They all need to be registered in the system, this is an important segment of document management from an auditor’s perspective. Since, for your compliance needs all relevant documentation has to be reviewed and approved by the relevant person (usually QMS manager or CEO), you need to show to the auditor ” The who, the how, and the when” did the review or approval. So, a good document management system should cover this issue with appropriate electronic signature-based approval implemented.
This is how it works in qmsWrapper:
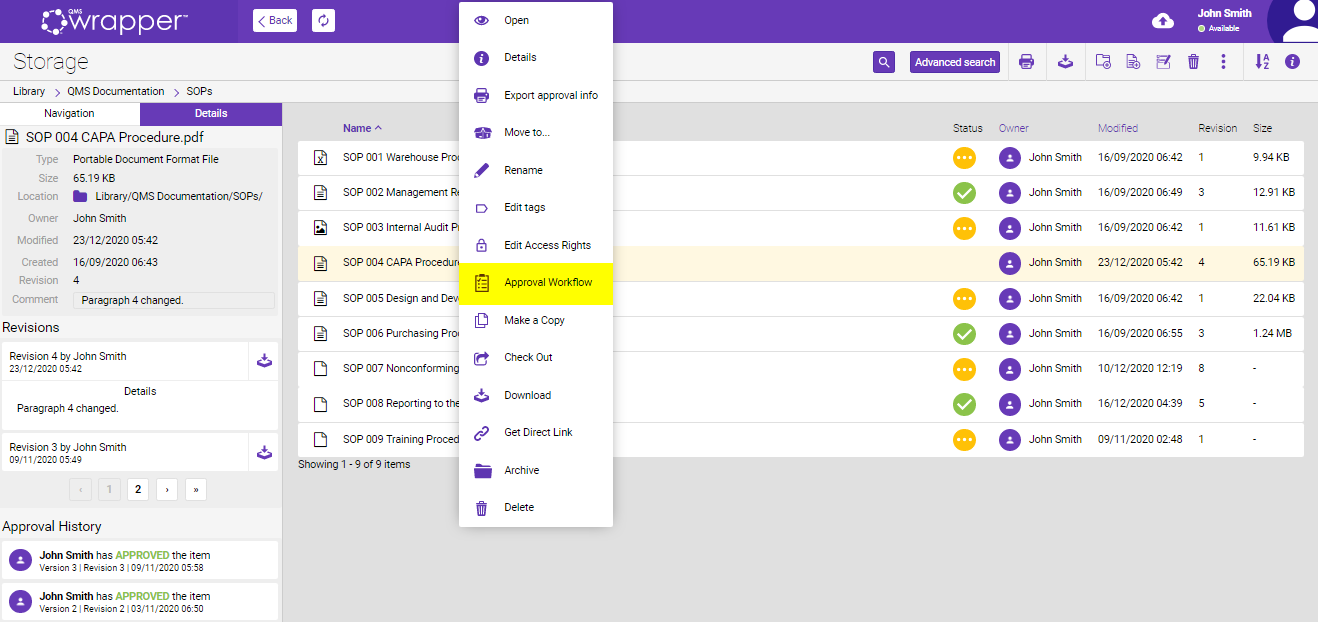
The file can be sent for approval using an “Approval Workflow” option. It is a feature that allows specific users to review and approve, or decline a document in the Storage. Files that are waiting for approval cannot be deleted, updated, or modified by any means.
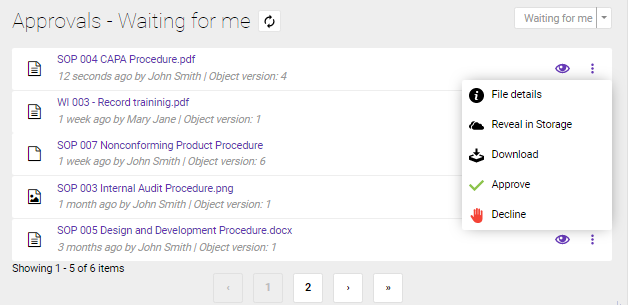
The document sent for approval, now appears on the designated user's Dashboard in the Documents approval – ‘Waiting for me’ section, from where it can be approved.
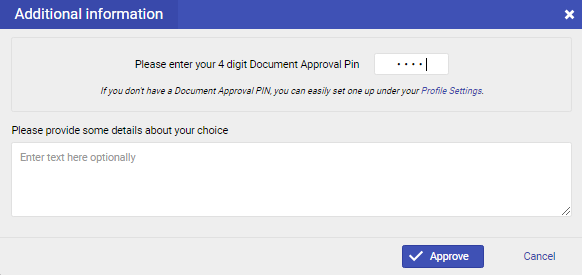
A file is approved with a 4-digit PIN that every user can set in the "Profile Settings".
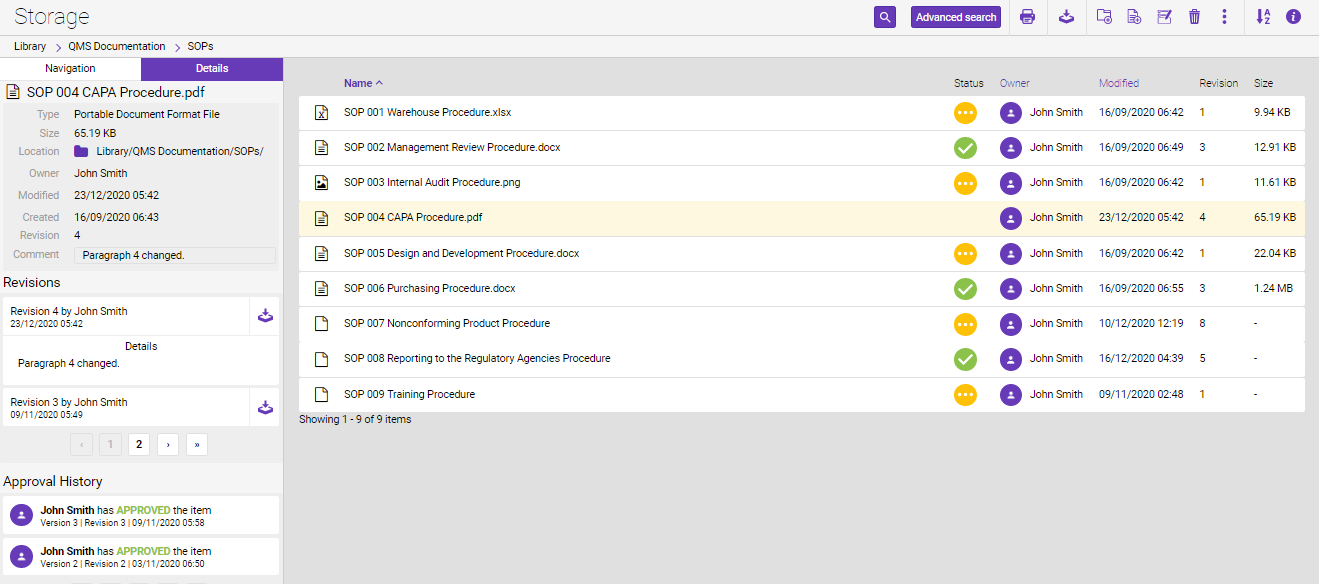
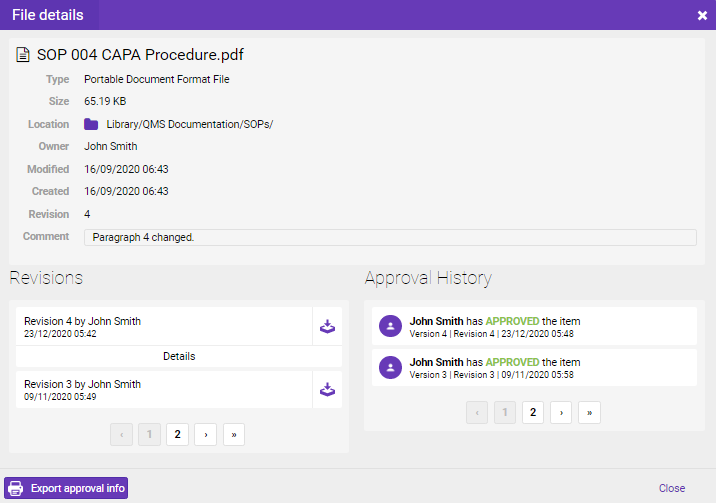
To view the Revision and Approval History of the file, you will need to go to the location folder of the file in the Storage, select the file, then “Details”.
Revisions section displays all file revisions with the comments. Each revision can be downloaded.
Approval History displays the approval status of the document and the person who approved the file. You are probably aware that besides the proper track of approval and review workflows, it is also a key segment to know who, when, and why the document was originated.
qmsWrapper has the functionality to record and track revision and approval history for every file that is uploaded to the Storage.
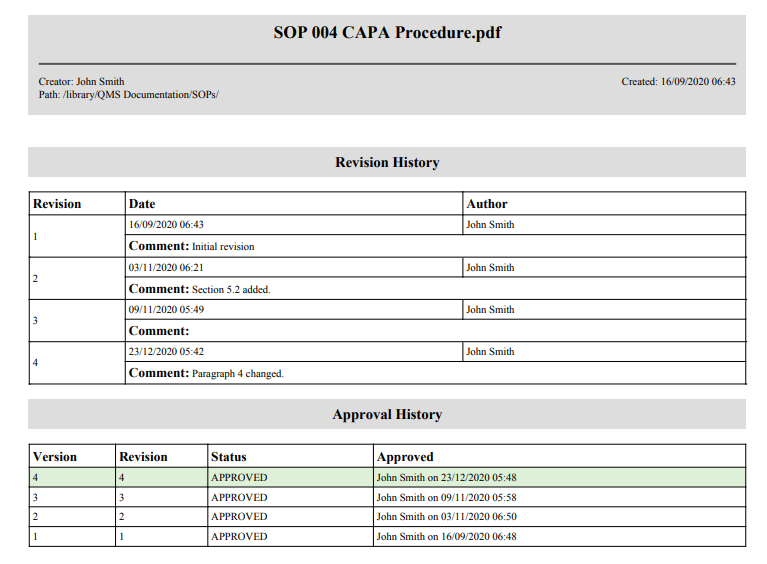
If it is necessary to provide these data to the external parties, they can be exported using the “Export approval info” option.
The “Export approval info” option creates a report about a file in PDF which contains general file information (name, size, type, owner, etc.), revision history data with comments, and information about approval status.
Have in mind that access rights to particular documents should be limited according to company or project role. No one should be able to see or edit everything, access rights should be adjustable by the main administrator of the software or by the relevant person, like the QMS manager.