
Jira is now “wrapped” with qmsWrapper
Now Medical Device startups and small businesses that use Jira can be connected to qmsWrapper. This integration will help medical device companies reduce their development risks and increase their agile effectiveness, whilst still keeping an eye on quality.
“qmsWrapper got its name because it originally designed to ‘wrap’ Jira in a QMS blanket, to take advantage of the best Agile development system and combine it, “wrap” it together with the best QMS system”
Drazenka Z. project manager
qmsWrapper is designed to be wrapped around Jira. This extends the benefits of Jira in the medical device world. Medical Devices startups and small businesses that use Jira can be linked to qmsWrapper.
Integration means better control of the development process.
The combination of Jira with qmsWrapper brings a system in which medical device manufacturers are able to track, manage and organize all CE Technical Files and FDA Design History Files,
and do it in the most effective manner possible.
This integration will help medical device companies reduce their development risks and increase their agile effectiveness, whilst still keeping an eye on quality.
Integration with Jira adds 3 main advantages:
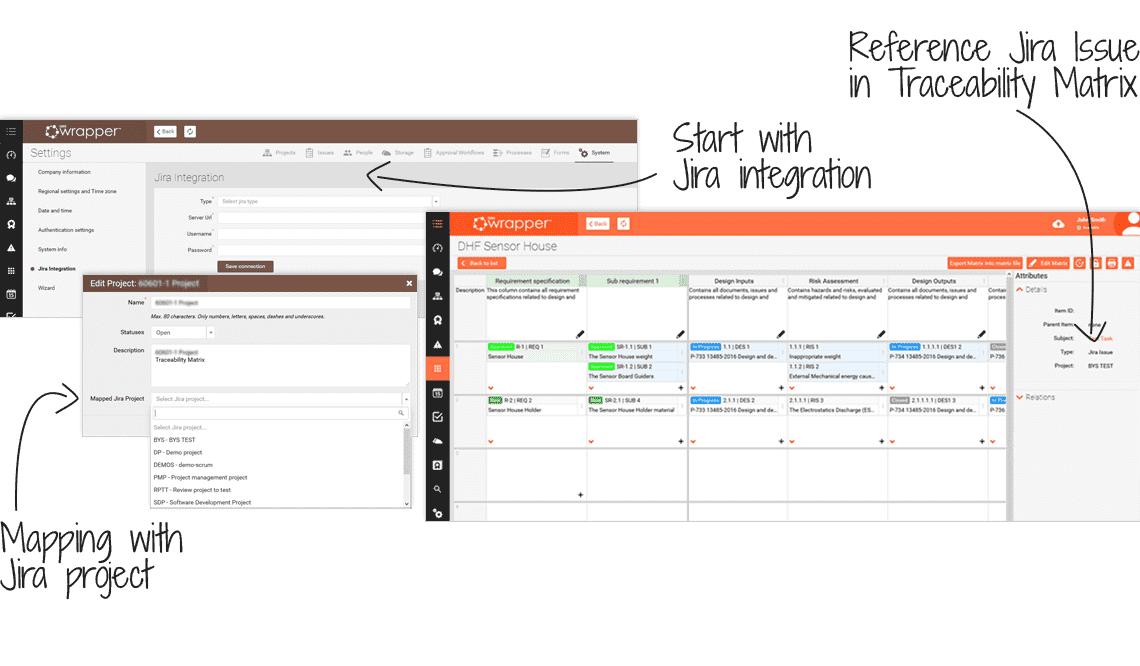
- Projects created in Jira are visible in qmsWrapper, they are connected.
- Issues created in Jira can be seen in qmsWrapper and tracked in Traceability Matrix.
- Connects a risk identified through Jira issues in the Traceability Matrix in a risk-related column.
Start integration with Jira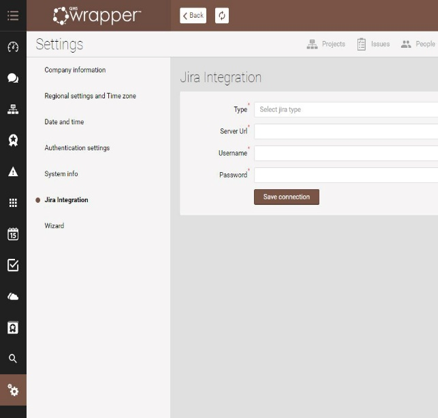
- General settings
- Jira integration
- Fill up the form
- Click “save integration”
Albert Malagarriga
|
We are looking for an eQMS solution that integrates nicely with our workflows, and noticed that you seem to do these implementations in Jira and Confluence.
We run on Jira (can add confluence) with integrations with github, and llm evaluation tools we are building in. We need to remain an agile software company deploying multiple times a day, so need heavy automation and a well greased machine, and are looking for external consulting to set this up for us. Can you help?