Weekly cup of Joe #2 - Documenting Compliance
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project… your strategy requires QMS oversight.
qmsJoe is here to guide you through the plethora of information and support you with handy tips & tricks to streamline your way to compliance.
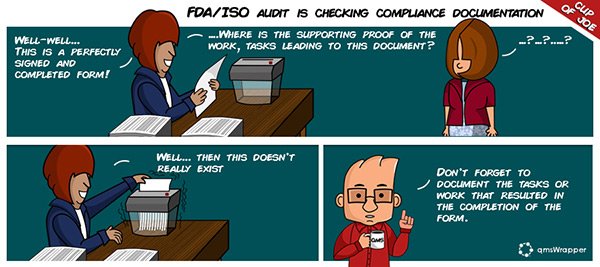
Simply having a signed document is no longer the standard of proof
For the FDA, the law is clear in 21 CFR 820.100 which states that “all activities required … and their results, shall be documented”. This is not a “should be” but a “shall be” requirement. FYI, it’s not optional. It is not enough to complete a signed QMS form saying the action was completed, any lack of documentation of QSR related activities or remedial actions, means that they never happened, signed form or not.
There are many examples where the FDA looked past the form to the work that should have been the end result of the produced work. If the tasks or work that should have resulted in the completion of the form is lacking, then FDA can simply conclude that there is inadequate proof as to the activities and their results were not documents. It does not mean you didn’t perform the QSR activities themselves, it means you didn’t document them, and as such to the FDA it is like these activities never happened.
In qmsWrapper any and all tasks, issues, documents can be tagged as a QMS event, that gets automatically reported to the QMS system, where not just project managers but any QMS manager can oversee the progress; CAPA can be followed back to its origin. Resolution with a signed document, electronic or otherwise, now has a date stamped electronic trail, that, if required, can be printed.
Management through quality is smart management that effectively avoids adding another layer of management by encouraging team-based collaboration compliance.
QMS Tags
Upgraded Dashboard and QMS Control
Wrapper File App – advanced editing and control of the documents