
A new Traceability Matrix so powerful, it becomes the products dashboard
TM is now more effective and useful feature that serves as a dashboard for product development leading to submission.
You build your Traceability Matrix as you develop your product...
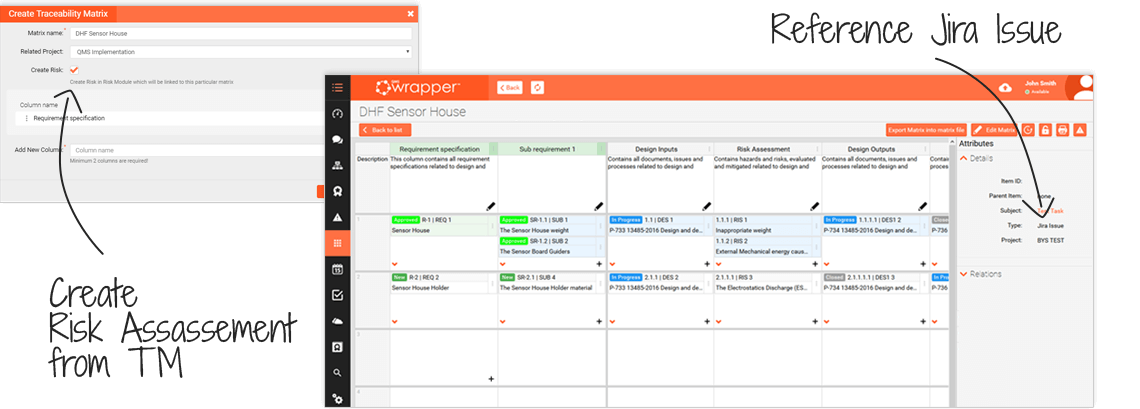
Key functions of qmsWrapper Traceability Matrix:
- track and show the relationships and connections between requirements
- end to end traceability
- design controls, by column
- tracking number automatically assigned and inherited
- export into a print-ready format for your FDA submission- it is your DHF
- integrated with document control and risk control
- integrated with processes, approvals and version controls
- integrated and synchronized with the project
- it's the cornerstone to your Life-Cycle processes

It helps track project requirements through steps that connect the dots.
Clearly demonstrates to inspectors/auditors the connection between the requirements, its design inputs, validation and verification, risk analysis, etc.
TRACEABILITY MATRIX IS FOUNDATION FOR YOUR DHF OR TECHNICAL FILE!
Notable features of Traceability Matrix?
- Multi-User implementation. All members of the team can work at the same time on the same TM
- Risk Assessment can be created from within the Traceability Matrix to go feature by feature
- Jira Integration - link Jira tasks, to testing, testing to validation
Traceability Matrix by qmsWrapper integrates with Jira, QMS, Project Management and Document Management. Requirements, specifications, design inputs and outputs, testing, life-cycle makes the core of DHF and TM and the backbone of 510k
or CE Mark submission.
Alcide
|
qmsWrapper
|
We are very happy to hear that because it confirms that we are doing good work. Waiting for your request :)
Ahmed
|
Thank you,
A.