
Cup of Joe 44# - Whose monitoring responsibility is when you have outsourced production?
When someone owns a medical device company, but not having a space or supplies for producing equipment, then they hire a contractor, outsourced manufacturer.
So, he is providing all the services regarding life-cycle products. If you think you've taken a heavy load off your back, you are wrong.
Please, read the regulations and standards again.
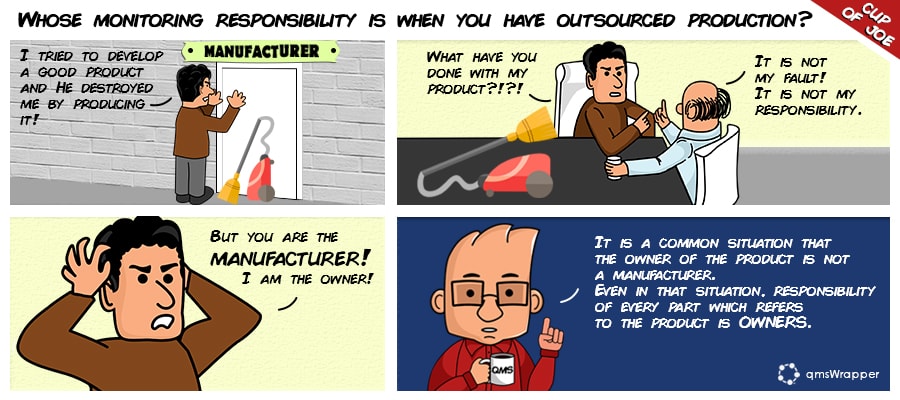
Let us remind you that it's said that if you choose to outsource any process that impacts contract manufacturer, it is your responsibility to monitor and ensure controls over the outsourced processes.
This includes defining roles and responsibilities in documented quality agreements with any outsourcing resources.
The owner retains all responsibility for the product. It means you still have to monitor and evaluate their system and fill up requirements.
Basically, there are few things that need to be doing from your side such as monitoring, evaluating, routine audits, management reviewal and simple check up on how the contractor is handling product issues.
Their must is to check with you before any changes are made to your device or in case some non-conformities are found.
Yours is to be kept informed on process handling and to make sure that goes along with QMS.
Stasha
|
Elvira A.
|