
Cup of Joe #47 - 2 Risk Modules
It’s always good to think a step ahead, and exactly that is what our Cup of Joe wants to tell you about.
qmsWrapper is constantly working on improvements and keeps up with the changes in the market that considers Medical Device startups and manufacturers.
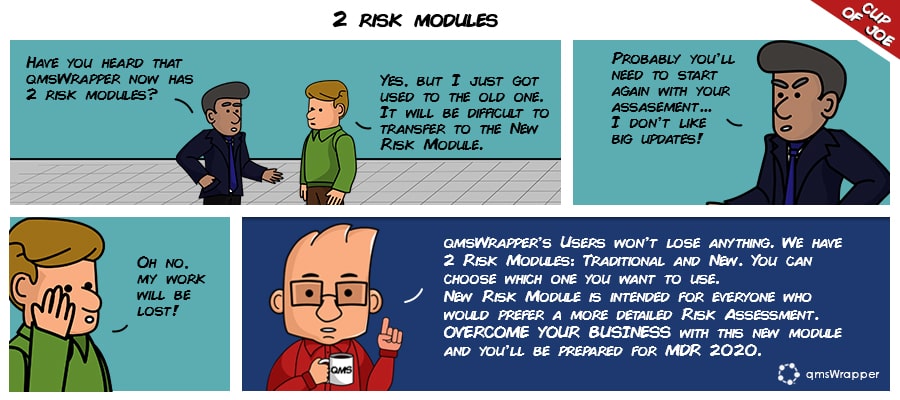
The new MDR is set to become applicable in May 2020. One of the changes is regarding the Risk Module, to be exact high-risk devices. This change is expected to reduce inconsistency in implementation between countries, given that the regulations supersede the laws of individual member states.
A new Risk Analysis Module (RAM), is introduced to help focus on the relationship between requirements, design, and benefits, with emphasis on risk-based design. It’s an updated version of ISO 14971:2019. It’s harmonized with new MDR requirements bringing a few changes in procedures that our upgraded software practically predicted. Upgrade still allows you to choose between the Traditional style Risk Analysis and the New Risk Module.
Alen
|
Bill
|
You have ISO 14971:2019 and 2014 version. So you can choose which one you want...
Please, someone, correct me if I am wrong