ISO 13485:2016 Summary
Medical Devices – Quality Management System – Requirements for Regulatory Purposes
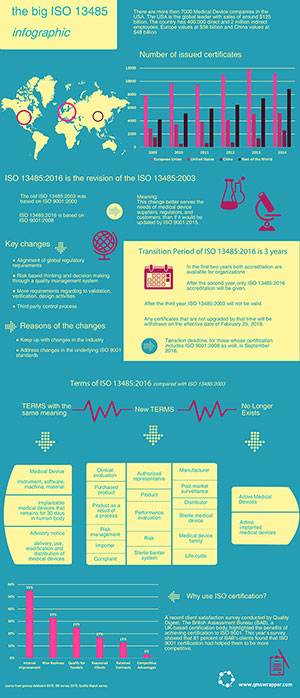
The world’s most popular standard for medical device quality management has been revised for the first time since 2003: Medical Devices – Quality Management System – Requirements for Regulatory Purposes.
The revised standard ISO 13485:2016 was published on 1st March 2016. It focuses on how companies should manage risk-based decisions related to purchasing, design, development, manufacturing, production control activities and other aspects of the quality management system.
Summary of the key changes
The ISO 13485 revision includes significant changes in a number of important areas. The following infographic offers a summary of these changes.
QMS solutions for small businesses or companies, much less startups, are few and far between. They are usually paper-based, or the better ones PDF-based and all are costly and cumbersome. But the qmsWrapper solution stands out, focusing not so much on the paperwork but on the processes from which ISO 13485:2003 and 2016 are founded upon, QMS processes.
By focusing on QMS processes, qmsWrapper targets how startups and small companies actually work, not adding to their work. We call it Management Through Quality (tm)or MTQ, our solution is using the aims of quality as management reporting points within the work process.
qmsWrapper published a team-based cooperative compliance approach that enables each member of the team to make their own contribution to compliance, such that the small efforts by each, accumulate to a systemic and more complete strategy. Rather than burden a few with all the responsibility of Regulatory and ISO standards compliance, MTQ empowers each member of the team to be a QMS contributor, a wrapper around compliance and QMS.
For Startups and smaller innovation companies qmsWrapper means they can address many of the requirements of ISO13485:2016, and of 2003 as well, without turning half the company into QMS managers.
Streamline the way you approach compliance, use qmsWrapper.
QMS Tags
Upgraded Dashboard and QMS Control
ISO 13485 implementation: Mandatory documents and records