
How To establish an ISO 13485:2016?
ISO 13485:2016 has set out requirements for a quality management system where you need to demonstrate your company’s ability to provide safe medical devices and related services that consistently meet customer and regulatory requirements.
Learn more about the certification flow to certificate through this infographic. It’s more eye-catching than texts, or in other words, more attention-drawing. But do not underestimate the quick and short lessons that we have prepared for your benefit
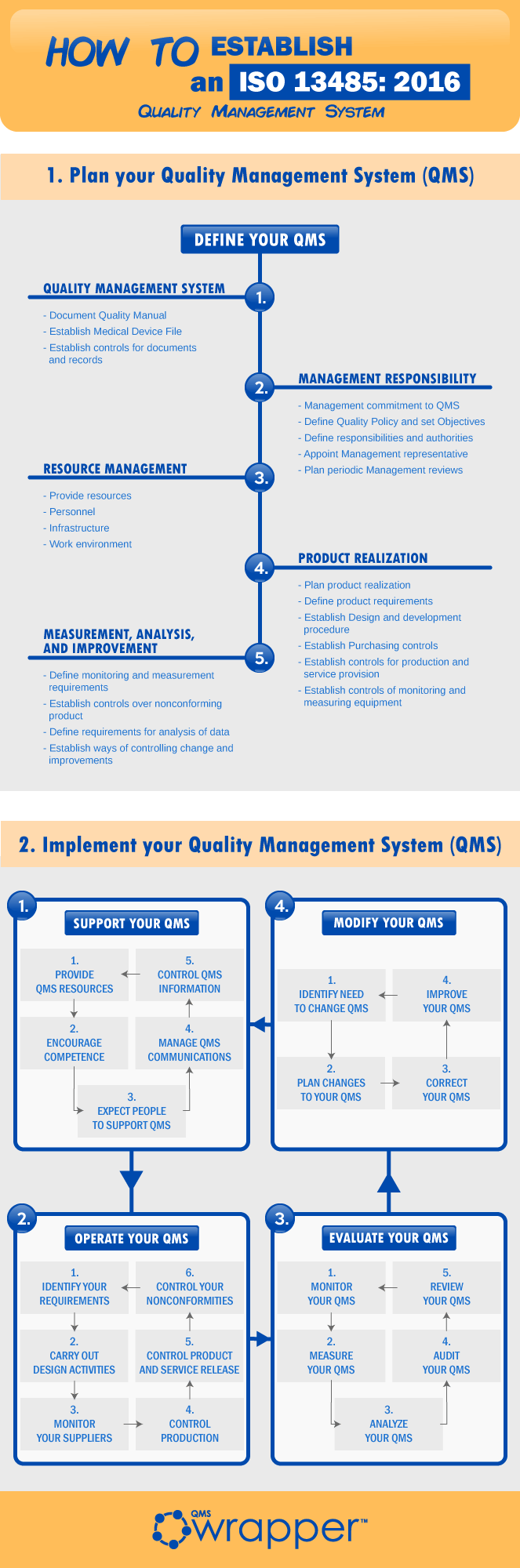
Never underestimate the value of the implementation of this ISO 13485:2016 standard because:
It shows clients and customers that your company takes quality very seriously and that you have a system in place to ensure it. When you use facts and data to drive your decisions tend to be better aligned with the strategic goals of your company.
Standard itself is a set of Quality management principles, one of which is ensuring customer satisfaction. The more your employees understand their roles in delivering quality products and services, the more engaged they are, which leads to increased efficiency and productivity. You’ll be able to identify and eliminate waste within and between processes, reduce errors, and avoid rework-facilitating greater efficiency and cost savings.
ISO 13485 implementation: Mandatory documents and records
Validation efforts
Gap analysis in QMS