Weekly Cup of Joe #26 – Advantages of An Integrated PM and QMS
Whether you are freshly minted into the QMS position or you are a founder of a Startup, or a product manager with a new project... your strategy requires QMS oversight.
QMS Joe will guide you with handy tips & tricks to streamline your way to compliance.
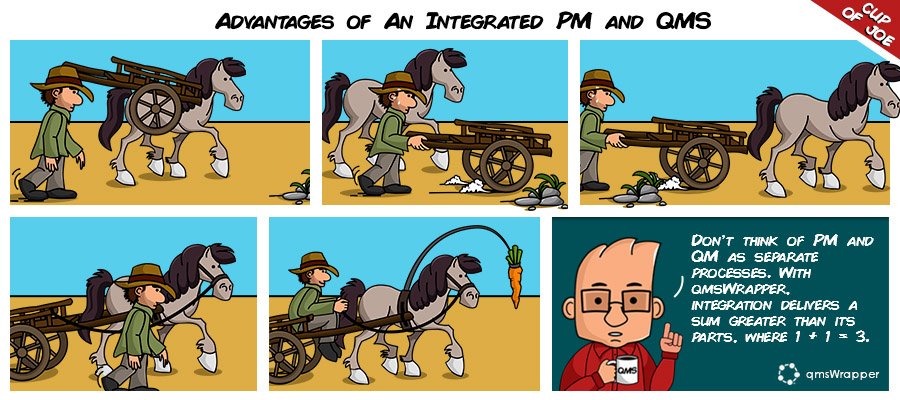
Bolt-on Quality Management Apps put the cart before the horse
The mistake we all make is that we all too often disassociate QMS from the real work environment, and real work is usually work that is Project-based, managed through some Project Management (PM) software.
Most companies simply “bolt-on” some QM System to their PM system but rarely are these understood by the users plodding through their PM based tasks.
The bolt-on QMS type of applications whether added to the PM app or duplicating is still a separate app – it’s not integrated.
They are usually in the form of a PDF workflow where a PDF form is pushed through a workflow from user to user. Users can’t understand when and why to trigger QM or compliance tasks, they have to have a high degree of knowledge of what to do and when.
Integrated PM & QMS; When 1 + 1 = 3
A.K.A. PM + QM = Compliance Management
Compliance can be a Team Collaboration effort! Rather than a bolt-on.
In integrated software, the modules are designed to work together, in a way to simplify the QMS steps for the user.
qmsWrapper is one such system.
One of the biggest features of qmsWrapper is that the defined QMS processes include all the ISO and FDA triggers built right in. Since it’s built PM style, all the project managers have to do (in order to ensure compliance) is to add the various Project tasks users have to accomplish. Now Project and QMS tasks are integrated into the same workflow process.
Furthermore, because QM Processes can be edited with the Process Editor, workflows can be changed and adapted to each company or project’s unique requirements, without changing the ISO or QMS triggers.
Now, when users complete their Project tasks, associated QM events will appear at the right time, in the right sequence, with the right instructions for completion, exactly like any other Project task.
And once completed, the QM Event will register not only within the Project but also within the QMS module so the QMS manager can also track it for compliance. The proof is now there and it is related to the work tasks that created it.
Additionally, integrated PM and QM include features such as QMS approval workflows, document history management, scheduling meetings, etc.
When the QM is integrated with the PM the results for the user are a much greater value than the mere sum of its parts, in this case, there is an argument that 1+1=3, or PM+QM=Compliance Management.
QMS Tags
Upgraded Dashboard and QMS Control
ISO 13485 implementation: Mandatory documents and records