Showing posts tagged with: risk management

How Companies Are Transforming Their Risk Assessment Processes for Better Results
Optimizing Medical Device Risk Management for Efficiency, Accuracy, and Compliance
One of the persistent challenges in medical device development is ensuring that risk assessment processes are both compliant and practically useful. While standards like ISO 14971 provide the framework, implementation in real-world scenarios often reveals inefficiencies — from inconsistent documentation to siloed decision-making.
Companies are now rethinking...

How to Apply AI Risk Prompts to Real-World Medical Device Development
Effective risk assessment is a foundation of compliant and safe medical device development. If you’ve downloaded our free guide with expert-level ChatGPT prompts, you've already made a strategic move toward improving your risk management processes.
Now it's time to translate those AI-generated insights into practical, real-world actions — grounded in standards like ISO 14971, IEC 60601,...

Cup of Joe #47 - 2 Risk Modules
It’s always good to think a step ahead, and exactly that is what our Cup of Joe wants to tell you about.
qmsWrapper is constantly working on improvements and keeps up with the changes in the market that considers Medical Device startups and manufacturers.
The new MDR is set to become applicable in May 2020. One of...

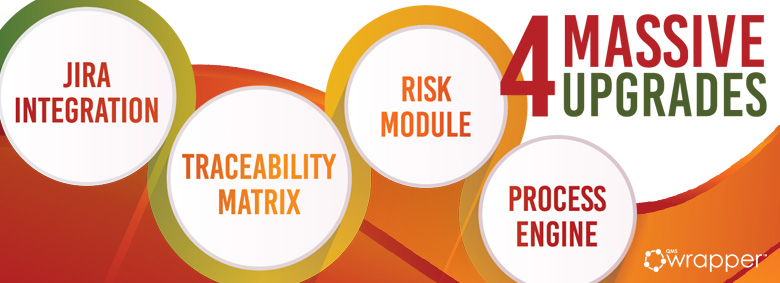
The New Risk Module supports risk-based design
Risk Analysis Module becomes a very important update, next to other features: Traceability Matrix, Process Engine, and Jira integration. Changes will make Risk Assessment much easier and clearer.
In the medical device world, risk-based design and development is the new norm. For FDA clearance and CE Mark certification, ISO14971 is essential.
And in May of 2020, the...
Wrap Jira in QMS
27th November 2019
Toronto, CA - QmsWrapper, a world-famous QMS System that helps Meddev startups and small businesses achieve and manage ISO 13485:2016 and 21 CFR 820, announces that the future is now available with the latest release where vision meets functionality.
Vs6.0 will radically change the way you look at QMS. It moves away from being...

How CAPA should be verified?
“Efficiency without Effectiveness misses the Purpose
Effectiveness without Efficiency misses all the Profit
Effectiveness with Efficiency brings Performance.”
― Martin U. Ugwu
Verifying the effectiveness of corrective and preventive actions (CAPAs) closes the loop between identifying a problem and completing the actions to solve it.
Reminder: The main purpose of corrective and preventive actions (CAPA) is to improve the organization processes...

Cup of Joe 43# - Treat the cause, not the symptom
What is the root cause analysis?
Root Cause Analysis is something that we all do when we want to solve a problem for good.
But what RCA actually means?
It means to find what caused the problem, to find out not only what went wrong in the beginning, but how, when and why as well.
Basically, RCA is...

CAPA Checklist - Step by Step Guide
CAPA – Corrective action Preventive action
The purpose of corrective and preventive action is to collect and inspect information, identify and examine the product or potential quality problems, then take suitable and effective corrective/preventive action.
It’s a process of improvement taken to eliminate causes of possible malfunction, problems, or other unwanted situations. It’s also a process that...

Weekly Cup of Joe #25– Documenting Opportunities to Improve
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project…your strategy requires QMS oversight.
MS Joe will guide you with handy tips & tricks to streamline your way to compliance.
The Relation Between Risk Assessment and Opportunities to Improve
Companies often fail to...

Weekly Cup of Joe #23– Corrective and Preventive Action (CAPA)
Whether you are freshly minted into the QMS position or you are a founder of a Startup, or a product manager with a new project… your strategy requires QMS oversight.
QMS Joe will guide you with handy tips & tricks to streamline your way to compliance.
Are Corrective Actions More Important Than Preventive Actions?
Although CAPA is...

Weekly Cup of Joe #22 – Risk Management Strategy in QMS
Whether you are freshly minted into the QMS position or you are a founder of a Startup, or a product manager with a new project… your strategy requires QMS oversight.
QMS Joe will guide you with handy tips & tricks to streamline your way to compliance.
Deer, Rabbits and Fast Snails, Risk is About Context
Risk management...
Hidden Pitfalls in the Risk Management Strategy
In our fast-paced world, the risks we have to take and manage, in order to continue to grow and to develop, evolve quickly. Effectively managed risks help companies achieve their goals.
Products that are developed following quality standards, have improved the quality of life for thousands of people. The idea of improving the quality of life...

Weekly Cup of Joe #13 – Strategy for Achieving Quality Objectives
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project… your strategy requires QMS oversight.
QMS Joe is here to guide you through the plethora of information and support you with handy tips & tricks to streamline your way to...

Weekly Cup of Joe #6 – Identifying potential hazards
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project… your strategy requires QMS oversight.
QMS Joe is here to guide you through the plethora of information and support you with handy tips & tricks to streamline your way to...

Risk as synonymous with uncertainty?
” Risk /rɪsk/, noun
the possibility that something unpleasant or unwelcome will happen.
Synonyms: chance, uncertainty, unpredictability, instability, riskiness…”
We’re dealing with countless risks in our daily life, like getting cut during apple slicing, or catching a cold if we get wet on a rainy day…or planning a project without a proper risk management plan…
Unknowingly you are doing...